Art B Why Does the Addition of Acid Increase the Solubility of Calcium Phosphate? Hints
Affiliate 15. Equilibria of Other Reaction Classes
15.i Precipitation and Dissolution
Learning Objectives
By the end of this section, you will be able to:
- Write chemical equations and equilibrium expressions representing solubility equilibria
- Carry out equilibrium computations involving solubility, equilibrium expressions, and solute concentrations
The preservation of medical laboratory blood samples, mining of sea h2o for magnesium, conception of over-the-counter medicines such as Milk of Magnesia and antacids, and treating the presence of hard water in your dwelling's water supply are just a few of the many tasks that involve controlling the equilibrium between a slightly soluble ionic solid and an aqueous solution of its ions.
In some cases, we desire to prevent dissolution from occurring. Molar decay, for example, occurs when the calcium hydroxylapatite, which has the formula Ca5(PO4)three(OH), in our teeth dissolves. The dissolution process is aided when leaner in our mouths feast on the sugars in our diets to produce lactic acid, which reacts with the hydroxide ions in the calcium hydroxylapatite. Preventing the dissolution prevents the decay. On the other manus, sometimes we desire a substance to dissolve. We desire the calcium carbonate in a chewable antacid to dissolve because the [latex]\text{CO}_3^{\;\;2-}[/latex] ions produced in this process help soothe an upset stomach.
In this section, nosotros will discover out how we tin control the dissolution of a slightly soluble ionic solid by the application of Le Châtelier's principle. Nosotros volition also learn how to use the equilibrium constant of the reaction to determine the concentration of ions present in a solution.
The Solubility Product Constant
Argent chloride is what'south known as a sparingly soluble ionic solid (Figure 1). Remember from the solubility rules in an earlier chapter that halides of Ag+ are not normally soluble. Even so, when we add an excess of solid AgCl to water, it dissolves to a small extent and produces a mixture consisting of a very dilute solution of Ag+ and Cl– ions in equilibrium with undissolved silver chloride:
[latex]\text{AgCl}(s)\;\underset{\text{atmospheric precipitation}}{\overset{\text{dissolution}}{\rightleftharpoons}}\;\text{Ag}^{+}(aq)\;+\;\text{Cl}^{-}(aq)[/latex]
This equilibrium, similar other equilibria, is dynamic; some of the solid AgCl continues to dissolve, but at the same time, Ag+ and Cl– ions in the solution combine to produce an equal corporeality of the solid. At equilibrium, the opposing processes accept equal rates.
The equilibrium constant for the equilibrium between a slightly soluble ionic solid and a solution of its ions is called the solubility product (Grand sp) of the solid. Recall from the chapter on solutions and colloids that we use an ion's concentration as an approximation of its activity in a dilute solution. For silver chloride, at equilibrium:
[latex]\text{AgCl}(s)\;{\rightleftharpoons}\;\text{Ag}^{+}(aq)\;+\;\text{Cl}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ag}^{+}(aq)][\text{Cl}^{-}(aq)][/latex]
When looking at dissolution reactions such as this, the solid is listed equally a reactant, whereas the ions are listed as products. The solubility product constant, every bit with every equilibrium constant expression, is written equally the product of the concentrations of each of the ions, raised to the ability of their stoichiometric coefficients. Here, the solubility product constant is equal to Ag+ and Cl– when a solution of silver chloride is in equilibrium with undissolved AgCl. There is no denominator representing the reactants in this equilibrium expression since the reactant is a pure solid; therefore [AgCl] does non appear in the expression for M sp.
Some mutual solubility products are listed in Tabular array 1 according to their Yard sp values, whereas a more all-encompassing compilation of products appears in Appendix J. Each of these equilibrium constants is much smaller than i because the compounds listed are merely slightly soluble. A pocket-sized 1000 sp represents a system in which the equilibrium lies to the left, so that relatively few hydrated ions would be present in a saturated solution.
| Substance | K sp at 25 °C |
|---|---|
| CuCl | 1.2 × 10–6 |
| CuBr | 6.27 × 10–ix |
| AgI | 1.5 × x–16 |
| PbS | 7 × ten–29 |
| Al(OH)three | 2 × 10–32 |
| Fe(OH)3 | 4 × 10–38 |
| Tabular array ane. Common Solubility Products by Decreasing Equilibrium Constants | |
Case ane
Writing Equations and Solubility Products
Write the ionic equation for the dissolution and the solubility product expression for each of the following slightly soluble ionic compounds:
(a) AgI, silverish iodide, a solid with antiseptic properties
(b) CaCOiii, calcium carbonate, the active ingredient in many over-the-counter chewable antacids
(c) Mg(OH)2, magnesium hydroxide, the agile ingredient in Milk of Magnesia
(d) Mg(NH4)POfour, magnesium ammonium phosphate, an essentially insoluble substance used in tests for magnesium
(e) Cafive(PO4)3OH, the mineral apatite, a source of phosphate for fertilizers
(Hint: When determining how to suspension (d) and (e) up into ions, refer to the list of polyatomic ions in the section on chemic classification.)
Solution
(a) [latex]\text{AgI}(southward)\;{\rightleftharpoons}\;\text{Ag}^{+}(aq)\;+\;\text{I}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ag}^{+}][\text{I}^{-}][/latex]
(b) [latex]\text{CaCO}_3(s)\;{\rightleftharpoons}\;\text{Ca}^{two+}(aq)\;+\;\text{CO}_3^{\;\;2-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ca}^{2+}][\text{CO}_3^{\;\;2-}][/latex]
(c) [latex]\text{Mg(OH)}_2(s)\;{\rightleftharpoons}\;\text{Mg}^{2+}(aq)\;+\;ii\text{OH}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Mg}^{2+}][\text{OH}^{-}]^2[/latex]
(d) [latex]\text{Mg(NH}_4)\text{PO}_4(south)\;{\rightleftharpoons}\;\text{Mg}^{2+}(aq)\;+\;\text{NH}_4^{\;\;+}(aq)\;+\;\text{PO}_4^{\;\;iii-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Mg}^{ii+}][\text{NH}_4^{\;\;+}][\text{PO}_4^{\;\;3-}][/latex]
(east) [latex]\text{Ca}_5(\text{PO}_4)3\text{OH}(south)\;{\rightleftharpoons}\;5\text{Ca}^{2+}(aq)\;+\;3\text{PO}_4^{\;\;3-}(aq)\;+\;\text{OH}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ca}^{2+}]^five[\text{PO}_4^{\;\;3-}]^3[\text{OH}^{-}][/latex]
Bank check Your Learning
Write the ionic equation for the dissolution and the solubility product for each of the following slightly soluble compounds:
(a) BaSO4
(b) Ag2SO4
(c) Al(OH)3
(d) Pb(OH)Cl
Reply:
(a) [latex]\text{BaSO}_4(due south)\;{\rightleftharpoons}\;\text{Ba}^{ii+}(aq)\;+\;\text{SO}_4^{\;\;ii-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ba}^{two+}][\text{SO}_4^{\;\;ii-}][/latex]; (b) [latex]\text{Ag}_2\text{SO}_4(due south)\;{\rightleftharpoons}\;two\text{Ag}^{+}(aq)\;+\;\text{So}_4^{\;\;2-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ag}^{+}]^ii[\text{And so}_4^{\;\;two-}][/latex]; (c) [latex]\text{Al(OH)}_3(southward)\;{\rightleftharpoons}\;\text{Al}^{two+}(aq)\;+\;3\text{OH}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Al}^{three+}][\text{OH}^{-}]^3[/latex]; (d) [latex]\text{Pb(OH)Cl}(south)\;{\rightleftharpoons}\;\text{Pb}^{2+}(aq)\;+\;\text{OH}^{-}(aq)\;+\;\text{Cl}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Atomic number 82}^{2+}][\text{OH}^{-}][\text{Cl}^{-}][/latex]
Now nosotros will extend the give-and-take of K sp and testify how the solubility production constant is determined from the solubility of its ions, as well every bit how K sp can be used to determine the molar solubility of a substance.
Chiliad sp and Solubility
Recall that the definition of solubility is the maximum possible concentration of a solute in a solution at a given temperature and pressure. Nosotros can decide the solubility product of a slightly soluble solid from that measure out of its solubility at a given temperature and pressure, provided that the merely pregnant reaction that occurs when the solid dissolves is its dissociation into solvated ions, that is, the simply equilibrium involved is:
[latex]\text{M}_p\text{X}_q(due south)\;{\rightleftharpoons}\;p\text{K}^{\text{m+}}(aq)\;+\;q\text{10}^{\text{n-}}(aq)[/latex]
In this case, nosotros calculate the solubility product past taking the solid'southward solubility expressed in units of moles per liter (mol/Fifty), known as its molar solubility.
Case 2
Calculation of M sp from Equilibrium Concentrations
We began the chapter with an informal discussion of how the mineral fluorite (Introduction to Affiliate fifteen) is formed. Fluorite, CaF2, is a slightly soluble solid that dissolves according to the equation:
[latex]\text{CaF}_2(s)\;{\rightleftharpoons}\;\text{Ca}^{2+}(aq)\;+\;ii\text{F}^{-}(aq)[/latex]
The concentration of Ca2+ in a saturated solution of CaF2 is ii.15 × 10–four Thousand; therefore, that of F– is iv.30 × 10–four M, that is, twice the concentration of Catwo+. What is the solubility production of fluorite?
Solution
First, write out the Grand sp expression, and then substitute in concentrations and solve for K sp:
[latex]\text{CaF}_2(south)\;{\rightleftharpoons}\;\text{Ca}^{two+}(aq)\;+\;2\text{F}^{-}(aq)[/latex]
A saturated solution is a solution at equilibrium with the solid. Thus:
[latex]K_{\text{sp}} = [\text{Ca}^{2+}][\text{F}^{-}]^2 = (2.1\;\times\;x^{-4})(4.2\;\times\;10^{-4})^2 = 3.7\;\times\;x^{-11}[/latex]
As with other equilibrium constants, we exercise not include units with K sp.
Bank check Your Learning
In a saturated solution that is in contact with solid Mg(OH)two, the concentration of Mg2+ is ane.31 × 10–4 G. What is the solubility production for Mg(OH)2?
[latex]\text{Mg(OH)}_2(s)\;{\rightleftharpoons}\;\text{Mg}^{two+}(aq)\;+\;2\text{OH}^{-}(aq)[/latex]
Example 3
Conclusion of Molar Solubility from K sp
The K sp of copper(I) bromide, CuBr, is 6.3 × 10–9. Calculate the molar solubility of copper bromide.
Solution
The solubility production constant of copper(I) bromide is vi.3 × 10–9.
The reaction is:
[latex]\text{CuBr}(s)\;{\rightleftharpoons}\;\text{Cu}^{+}(aq)\;+\;\text{Br}^{-}(aq)[/latex]
Starting time, write out the solubility product equilibrium constant expression:
[latex]K_{\text{sp}} = [\text{Cu}^{+}][\text{Br}^{-}][/latex]
Create an Water ice table (as introduced in the chapter on key equilibrium concepts), leaving the CuBr cavalcade empty equally it is a solid and does not contribute to the Thousand sp:
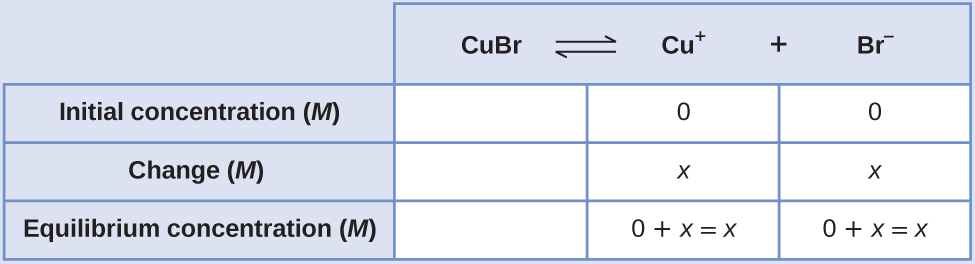
At equilibrium:
[latex]K_{\text{sp}} = [\text{Cu}^{+}][\text{Br}^{-}][/latex]
[latex]6.three\;\times\;10^{-ix} = (10)(10) = x^ii[/latex]
[latex]10 = \sqrt{(6.iii\;\times\;x^{-9})} = 7.ix\;\times\;10^{-5}[/latex]
Therefore, the molar solubility of CuBr is 7.9 × 10–5 M.
Bank check Your Learning
The Yard sp of AgI is 1.5 × 10–16. Calculate the molar solubility of silver iodide.
Case four
Determination of Molar Solubility from K sp, Part II
The 1000 sp of calcium hydroxide, Ca(OH)2, is 1.3 × x–6. Summate the molar solubility of calcium hydroxide.
Solution
The solubility production constant of calcium hydroxide is 1.3 × x–6.
The reaction is:
[latex]\text{Ca(OH)}_2(s)\;{\rightleftharpoons}\;\text{Ca}^{two+}(aq)\;+\;2\text{OH}^{-}(aq)[/latex]
First, write out the solubility product equilibrium constant expression:
[latex]K_{\text{sp}} = [\text{Ca}^{2+}][\text{OH}^{-}]^2[/latex]
Create an Water ice tabular array, leaving the Ca(OH)2 column empty every bit it is a solid and does not contribute to the M sp:
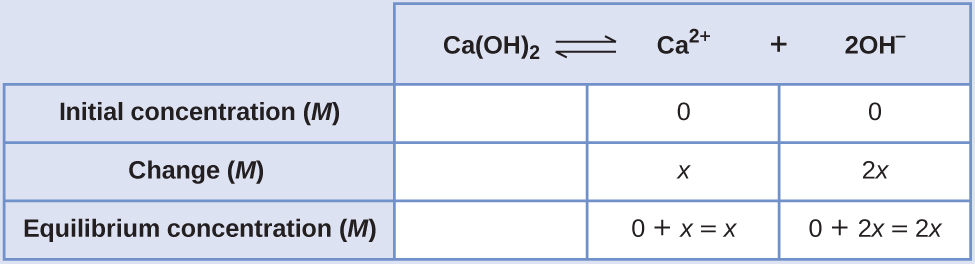
At equilibrium:
[latex]K_{\text{sp}} = [\text{Ca}^{ii+}][\text{OH}^{-}]^2[/latex]
[latex]i.iii\;\times\;10^{-6} = (x)(2x)^two = (x)(4x^ii) = 4x^iii[/latex]
[latex]10 = \sqrt[3]{\frac{1.iii\;\times\;ten^{-6}}{4}} = 6.nine\;\times\;10^{-iii}[/latex]
Therefore, the molar solubility of Ca(OH)2 is i.three × 10–2 M.
Cheque Your Learning
The K sp of PbI2 is 1.4 × x–viii. Summate the molar solubility of lead(Ii) iodide.
Note that solubility is not ever given equally a tooth value. When the solubility of a compound is given in some unit other than moles per liter, nosotros must catechumen the solubility into moles per liter (i.eastward., molarity) in order to apply it in the solubility product abiding expression. Case 5 shows how to perform those unit of measurement conversions before determining the solubility production equilibrium.
Instance 5
Determination of K sp from Gram Solubility
Many of the pigments used past artists in oil-based paints (Figure two) are sparingly soluble in water. For case, the solubility of the artist's pigment chrome xanthous, PbCrO4, is 4.6 × 10–6 thou/L. Decide the solubility production equilibrium constant for PbCrOiv.

Solution
We are given the solubility of PbCrO4 in grams per liter. If we convert this solubility into moles per liter, we tin observe the equilibrium concentrations of Pb2+ and [latex]\text{CrO}_4^{\;\;2-}[/latex], then Thousand sp:
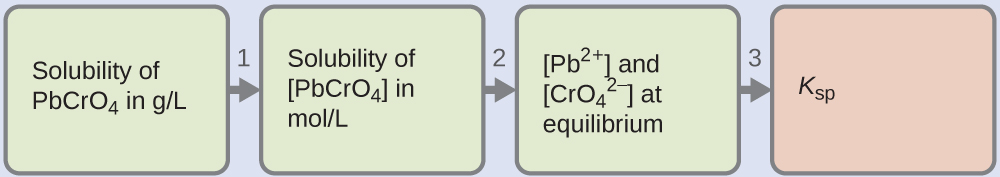
- Apply the molar mass of PbCrOfour [latex](\frac{323.2\;\text{thou}}{1\;\text{mol}})[/latex] to catechumen the solubility of PbCrOiv in grams per liter into moles per liter:
[latex][\text{PbCrO}_4] = \frac{iv.6\;\times\;ten^{-6}\;\text{g}\;\text{PbCrO}_4}{i\;\text{L}}\;\times\;\frac{ane\;\text{mol}\;\text{PbCrO}_4}{323.2\;\text{g\;PbCrO}_4}[/latex]
[latex]= \frac{1.4\;\times\;ten^{-8}\;\text{mol\;PbCrO}_4}{1\;\text{L}}[/latex]
[latex]= ane.4\;\times\;10^{-8}\;M[/latex] - The chemical equation for the dissolution indicates that 1 mol of PbCrO4 gives 1 mol of Pb2+(aq) and ane mol of [latex]\text{CrO}_4^{\;\;2-}(aq)[/latex]:
[latex]\text{PbCrO}_4(southward)\;{\rightleftharpoons}\;\text{Atomic number 82}^{two+}(aq)\;+\;\text{CrO}_4^{\;\;ii-}(aq)[/latex]
Thus, both [Lead2+] and [latex][\text{CrO}_4^{\;\;2-}][/latex] are equal to the molar solubility of PbCrOfour:
[latex][\text{Pb}^{2+}] = [\text{CrO}_4^{\;\;2-}] = i.four\;\times\;ten^{-viii}\;K[/latex]
- Solve. One thousand sp = [Lead2+][latex][\text{CrO}_4^{\;\;ii-}][/latex] = (one.4 × 10–8)(ane.4 × ten–viii) = 2.0 × ten–sixteen
Check Your Learning
The solubility of TlCl [thallium(I) chloride], an intermediate formed when thallium is being isolated from ores, is 3.46 grams per liter at 20 °C. What is its solubility product?
Case half dozen
Calculating the Solubility of Hg2Clii
Calomel, Hg2Clii, is a compound equanimous of the diatomic ion of mercury(I), [latex]\text{Hg}_2^{\;\;2+}[/latex], and chloride ions, Cl–. Although near mercury compounds are at present known to exist poisonous, eighteenth-century physicians used calomel as a medication. Their patients rarely suffered any mercury poisoning from the treatments because calomel is quite insoluble:
[latex]\text{Hg}_2\text{Cl}_2(s)\;{\rightleftharpoons}\;\text{Hg}_2^{\;\;ii+}(aq)\;+\;two\text{Cl}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = one.1\;\times\;10^{-18}[/latex]
Calculate the molar solubility of Hg2Cltwo.
Solution
The molar solubility of Hg2Cl2 is equal to the concentration of [latex]\text{Hg}_2^{\;\;ii+}[/latex] ions because for each one mol of HgtwoCl2 that dissolves, 1 mol of [latex]\text{Hg}_2^{\;\;2+}[/latex] forms:
- Determine the management of alter. Before any Hg2Cltwo dissolves, Q is zero, and the reaction will shift to the right to achieve equilibrium.
- Make up one's mind x and equilibrium concentrations. Concentrations and changes are given in the following Ice table:
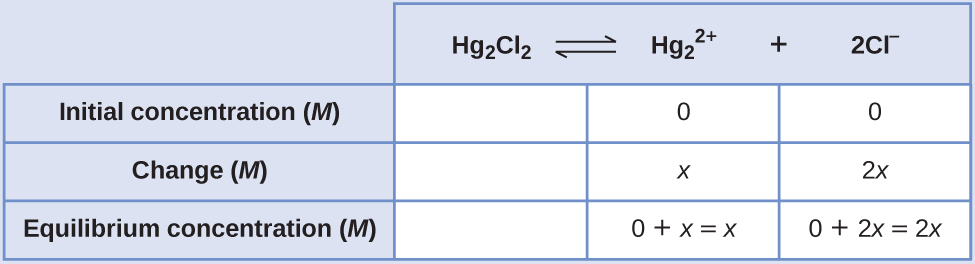
Note that the alter in the concentration of Cl– (2x) is twice as big equally the alter in the concentration of [latex]\text{Hg}_2^{\;\;2+}[/latex] (10) because 2 mol of Cl– forms for each 1 mol of [latex]\text{Hg}_2^{\;\;ii+}[/latex] that forms. HgiiCl2 is a pure solid, so it does not appear in the calculation.
- Solve for ten and the equilibrium concentrations. We substitute the equilibrium concentrations into the expression for 1000 sp and summate the value of 10:
[latex]K_{\text{sp}} = [\text{Hg}_2^{\;\;2+}][\text{Cl}^{-}]^ii[/latex]
[latex]1.ane\;\times\;10^{-eighteen} = (ten)(2x)^2[/latex]
[latex]4x^3 = one.1\;\times\;10^{-18}[/latex]
[latex]10 = \sqrt[3]{(\frac{one.1\;\times\;10^{-xviii}}{4})} = six.v\;\times\;10^{-7}\;M[/latex]
[latex][\text{Hg}_2^{\;\;2+}] = vi.5\;\times\;10^{-vii}\;1000 = 6.5\;\times\;x^{-7}\;M[/latex]
[latex][\text{Cl}^{-}] = 2x = 2(half dozen.5\;\times\;10^{-7}) = 1.iii\;\times\;10^{-6}\;M[/latex]
The tooth solubility of HgtwoCl2 is equal to [latex][\text{Hg}_2^{\;\;ii+}][/latex], or 6.v × x–7 Chiliad.
- Check the piece of work. At equilibrium, Q = K sp:
[latex]Q = [\text{Hg}_2^{\;\;2+}][\text{Cl}^{-}]^2 = (6.five\;\times\;10^{-vii})(ane.3\;\times\;ten^{-6})^2 = i.1\;\times\;10^{-18}[/latex]
The calculations check.
Check Your Learning
Determine the tooth solubility of MgF2 from its solubility product: K sp = 6.4 × x–9.
Tabulated K sp values can also exist compared to reaction quotients calculated from experimental data to tell whether a solid will precipitate in a reaction under specific atmospheric condition: Q equals K sp at equilibrium; if Q is less than K sp, the solid will dissolve until Q equals K sp; if Q is greater than K sp, atmospheric precipitation will occur at a given temperature until Q equals Yard sp.
Using Barium Sulfate for Medical Imaging
Diverse types of medical imaging techniques are used to aid diagnoses of illnesses in a noninvasive manner. One such technique utilizes the ingestion of a barium compound before taking an 10-ray epitome. A suspension of barium sulfate, a chalky pulverization, is ingested by the patient. Since the M sp of barium sulfate is one.1 × 10–10, very little of information technology dissolves every bit it coats the lining of the patient's intestinal tract. Barium-coated areas of the digestive tract and so appear on an X-ray equally white, allowing for greater visual detail than a traditional X-ray (Effigy 3).
Further diagnostic testing can be done using barium sulfate and fluoroscopy. In fluoroscopy, a continuous X-ray is passed through the torso so the physician tin monitor, on a TV or computer screen, the barium sulfate's motion as it passes through the digestive tract. Medical imaging using barium sulfate can be used to diagnose acid reflux disease, Crohn's disease, and ulcers in addition to other atmospheric condition.
Visit this website for more information on how barium is used in medical diagnoses and which conditions information technology is used to diagnose.
Predicting Precipitation
The equation that describes the equilibrium betwixt solid calcium carbonate and its solvated ions is:
[latex]\text{CaCO}_3(s)\;{\rightleftharpoons}\;\text{Ca}^{2+}(aq)\;+\;\text{CO}_3^{\;\;two-}(aq)[/latex]
Nosotros can constitute this equilibrium either past adding solid calcium carbonate to water or by mixing a solution that contains calcium ions with a solution that contains carbonate ions. If we add calcium carbonate to h2o, the solid will dissolve until the concentrations are such that the value of the reaction quotient [latex](\text{Q} = [\text{Ca}^{2+}][\text{CO}_3^{\;\;2-}])[/latex] is equal to the solubility product (Grand sp = 8.7 × x–nine). If we mix a solution of calcium nitrate, which contains Ca2+ ions, with a solution of sodium carbonate, which contains [latex]\text{CO}_3^{\;\;2-}[/latex] ions, the slightly soluble ionic solid CaCO3 will precipitate, provided that the concentrations of Caii+ and [latex]\text{CO}_3^{\;\;ii-}[/latex] ions are such that Q is greater than K sp for the mixture. The reaction shifts to the left and the concentrations of the ions are reduced by germination of the solid until the value of Q equals G sp. A saturated solution in equilibrium with the undissolved solid volition result. If the concentrations are such that Q is less than Thousand sp, then the solution is not saturated and no precipitate volition form.
Nosotros can compare numerical values of Q with K sp to predict whether precipitation will occur, every bit Example 7 shows. (Annotation: Since all forms of equilibrium constants are temperature dependent, we will presume a room temperature environs going frontwards in this chapter unless a different temperature value is explicitly specified.)
Instance 7
Precipitation of Mg(OH)2
The offset stride in the preparation of magnesium metal is the atmospheric precipitation of Mg(OH)2 from sea water past the addition of lime, Ca(OH)2, a readily available cheap source of OH– ion:
[latex]\text{Mg(OH)}_2(s)\;{\rightleftharpoons}\;\text{Mg}^{two+}(aq)\;+\;2\text{OH}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = 8.9\;\times\;10^{-12}[/latex]
The concentration of Mg2+(aq) in sea water is 0.0537 M. Volition Mg(OH)2 precipitate when enough Ca(OH)2 is added to give a [OH–] of 0.0010 M?
Solution
This problem asks whether the reaction:
[latex]\text{Mg(OH)}_2(southward)\;{\leftrightharpoons}\;\text{Mg}^{2+}(aq)\;+\;2\text{OH}^{-}(aq)[/latex]
shifts to the left and forms solid Mg(OH)2 when [Mg2+] = 0.0537 M and [OH–] = 0.0010 Thousand. The reaction shifts to the left if Q is greater than Grand sp. Adding of the reaction quotient under these weather is shown here:
[latex]Q = [\text{Mg}^{ii+}][\text{OH}^{-}]^2 = (0.0537)(0.0010)^2 = 5.4\;\times\;10^{-8}[/latex]
Because Q is greater than K sp (Q = 5.4 × 10–viii is larger than K sp = 8.9 × 10–12), we can look the reaction to shift to the left and form solid magnesium hydroxide. Mg(OH)two(s) forms until the concentrations of magnesium ion and hydroxide ion are reduced sufficiently so that the value of Q is equal to K sp.
Check Your Learning
Use the solubility product in Appendix J to determine whether CaHPO4 volition precipitate from a solution with [Ca2+] = 0.0001 G and [latex][\text{HPO}_4^{\;\;2-}][/latex] = 0.001 M.
Answer:
No precipitation of CaHPOiv; Q = one × 10–vii, which is less than K sp
Example 8
Precipitation of AgCl upon Mixing Solutions
Does silver chloride precipitate when equal volumes of a 2.0 × x–4–One thousand solution of AgNO3 and a two.0 × x–4–M solution of NaCl are mixed?
(Note: The solution also contains Na+ and [latex]\text{NO}_3^{\;\;-}[/latex] ions, merely when referring to solubility rules, one can meet that sodium nitrate is very soluble and cannot form a precipitate.)
Solution
The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is:
[latex]\text{AgCl}(due south)\;{\rightleftharpoons}\;\text{Ag}^{+}(aq)\;+\;\text{Cl}^{-}(aq)[/latex]
The solubility product is one.half dozen × 10–10 (see Appendix J).
AgCl volition precipitate if the reaction quotient calculated from the concentrations in the mixture of AgNOiii and NaCl is greater than Thousand sp. The volume doubles when we mix equal volumes of AgNOthree and NaCl solutions, so each concentration is reduced to half its initial value. Consequently, immediately upon mixing, [Ag+] and [Cl–] are both equal to:
[latex]\frac{one}{2}(2.0\;\times\;10^{-4})\;M = 1.0\;\times\;10^{-iv}\;Grand[/latex]
The reaction caliber, Q, is momentarily greater than K sp for AgCl, so a supersaturated solution is formed:
[latex]Q = [\text{Ag}^{+}][\text{Cl}^{-}] = (1.0\;\times\;10^{-4})(i.0\;\times\;10^{-four}) = 1.0\;\times\;10^{-8}\;{\textgreater}\;K_{\text{sp}}[/latex]
Since supersaturated solutions are unstable, AgCl will precipitate from the mixture until the solution returns to equilibrium, with Q equal to K sp.
Check Your Learning
Will KClO4 precipitate when xx mL of a 0.050-M solution of Grand+ is added to fourscore mL of a 0.50-1000 solution of [latex]\text{ClO}_4^{\;\;-}[/latex]? (Remember to calculate the new concentration of each ion later mixing the solutions earlier plugging into the reaction caliber expression.)
Answer:
No, Q = 4.0 × 10–iii, which is less than K sp = 1.05 × 10–2
In the previous two examples, we have seen that Mg(OH)2 or AgCl precipitate when Q is greater than 1000 sp. In full general, when a solution of a soluble salt of the Chiliadm+ ion is mixed with a solution of a soluble salt of the Xn– ion, the solid, Thou p X q precipitates if the value of Q for the mixture of Yardm+ and 10n– is greater than Grand sp for M p 10 q . Thus, if we know the concentration of 1 of the ions of a slightly soluble ionic solid and the value for the solubility product of the solid, then we can calculate the concentration that the other ion must exceed for precipitation to brainstorm. To simplify the calculation, nosotros will presume that atmospheric precipitation begins when the reaction quotient becomes equal to the solubility product constant.
Example 9
Precipitation of Calcium Oxalate
Claret will non clot if calcium ions are removed from its plasma. Some blood collection tubes contain salts of the oxalate ion, [latex]\text{C}_2\text{O}_4^{\;\;2-}[/latex], for this purpose (Figure 4). At sufficiently high concentrations, the calcium and oxalate ions form solid, CaCtwoOfour·H2O (which also contains water bound in the solid). The concentration of Ca2+ in a sample of blood serum is 2.2 × 10–3 Thousand. What concentration of [latex]\text{C}_2\text{O}_4^{\;\;2-}[/latex] ion must be established before CaC2O4·H2O begins to precipitate?
Solution
The equilibrium expression is:
[latex]\text{CaC}_2\text{O}_4(s)\;{\rightleftharpoons}\;\text{Ca}^{two+}(aq)\;+\;\text{C}_2\text{O}_4^{\;\;2-}(aq)[/latex]
For this reaction:
[latex]K_{\text{sp}} = [\text{Ca}^{2+}][\text{C}_2\text{O}_4^{\;\;ii-}] = 1.96\;\times\;x^{-viii}[/latex]
(encounter Appendix J)
CaC2O4 does not appear in this expression considering information technology is a solid. H2o does not appear because information technology is the solvent.
Solid CaC2O4 does not brainstorm to grade until Q equals Thousand sp. Because nosotros know K sp and [Ca2+], we can solve for the concentration of [latex]\text{C}_2\text{O}_4^{\;\;ii-}[/latex] that is necessary to produce the kickoff trace of solid:
[latex]Q = K_{\text{sp}} = [\text{Ca}^{ii+}][\text{C}_2\text{O}_4^{\;\;2-}] = ane.96\;\times\;10^{-8}[/latex]
[latex](2.2\;\times\;10^{-iii})[\text{C}_2\text{O}_4^{\;\;two-}] = ane.96\;\times\;10^{-viii}[/latex]
[latex][\text{C}_2\text{O}_4^{\;\;2-}] = \frac{1.96\;\times\;ten^{-8}}{2.2\;\times\;10^{-3}} = 8.9\;\times\;ten^{-6}[/latex]
A concentration of [latex][\text{C}_2\text{O}_4^{\;\;2-}][/latex] = eight.ix × 10–vi M is necessary to initiate the atmospheric precipitation of CaC2O4 under these conditions.
Bank check Your Learning
If a solution contains 0.0020 mol of [latex]\text{CrO}_4^{\;\;2-}[/latex] per liter, what concentration of Ag+ ion must be reached by adding solid AgNO3 before AgtwoCrOiv begins to precipitate? Fail any increase in volume upon calculation the solid silvery nitrate.
It is sometimes useful to know the concentration of an ion that remains in solution after precipitation. We can apply the solubility production for this adding besides: If we know the value of K sp and the concentration of one ion in solution, we can calculate the concentration of the 2d ion remaining in solution. The calculation is of the same type as that in Example nine—calculation of the concentration of a species in an equilibrium mixture from the concentrations of the other species and the equilibrium constant. However, the concentrations are different; we are computing concentrations later on precipitation is complete, rather than at the start of precipitation.
Example ten
Concentrations Following Precipitation
Wear washed in water that has a manganese [Mn2+(aq)] concentration exceeding 0.1 mg/L (ane.8 × ten–6 M) may be stained by the manganese upon oxidation, but the amount of Mn2+ in the h2o tin can be reduced by calculation a base. If a person doing laundry wishes to add a buffer to go along the pH high enough to precipitate the manganese as the hydroxide, Mn(OH)two, what pH is required to keep [Mntwo+] equal to 1.8 × 10–6 1000?
Solution
The dissolution of Mn(OH)2 is described by the equation:
[latex]\text{Mn(OH)}_2(south)\;{\rightleftharpoons}\;\text{Mn}^{2+}(aq)\;+\;2\text{OH}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = 2\;\times\;ten^{-three}[/latex]
Nosotros need to calculate the concentration of OH– when the concentration of Mn2+ is 1.viii × 10–6 M. From that, we summate the pH. At equilibrium:
[latex]K_{\text{sp}} = [\text{Mn}^{2+}][\text{OH}^{-}]^two[/latex]
or
[latex](one.8\;\times\;x^{-6})[\text{OH}^{-}]^2 = two\;\times\;x^{-iii}[/latex]
so
[latex][\text{OH}^{-}] = 3.3\;\times\;ten^{-4}\;G[/latex]
Now nosotros calculate the pH from the pOH:
[latex]\text{pOH} = -\text{log}[\text{OH}^{-}] = -\text{log}(3.3\;\times\;10\;-\;iv) = iii.48[/latex]
[latex]\text{pH} = 14.00\;-\;\text{pOH} = 14.00\;-\;3.80 = 10.52[/latex]
If the person doing laundry adds a base of operations, such equally the sodium silicate (Na4SiO4) in some detergents, to the wash water until the pH is raised to ten.52, the manganese ion will be reduced to a concentration of 1.8 × 10–six Grand; at that concentration or less, the ion volition not stain clothing.
Check Your Learning
The first pace in the training of magnesium metal is the precipitation of Mg(OH)ii from ocean water by the addition of Ca(OH)2. The concentration of Mg2+(aq) in sea water is 5.37 × 10–2 M. Calculate the pH at which [Mg2+] is diminished to 1.0 × 10–5 M by the improver of Ca(OH)2.
Due to their lite sensitivity, mixtures of silverish halides are used in fiber optics for medical lasers, in photochromic eyeglass lenses (glass lenses that automatically darken when exposed to sunlight), and—before the advent of digital photography—in photographic film. Even though AgCl (K sp = 1.6 × 10–10), AgBr (K sp = 5.0 × 10–xiii), and AgI (K sp = 1.v × ten–16) are each quite insoluble, we cannot fix a homogeneous solid mixture of them by calculation Ag+ to a solution of Cl–, Br–, and I–; essentially all of the AgI volition precipitate earlier whatever of the other solid halides course because of its smaller value for K sp. Nevertheless, we can prepare a homogeneous mixture of the solids by slowly adding a solution of Cl–, Br–, and I– to a solution of Ag+.
When two anions form slightly soluble compounds with the same cation, or when two cations class slightly soluble compounds with the same anion, the less soluble chemical compound (unremarkably, the compound with the smaller K sp) by and large precipitates first when we add a precipitating agent to a solution containing both anions (or both cations). When the K sp values of the two compounds differ past 2 orders of magnitude or more than (e.1000., 10–2 vs. x–4), well-nigh all of the less soluble chemical compound precipitates before any of the more soluble one does. This is an example of selective precipitation, where a reagent is added to a solution of dissolved ions causing one of the ions to precipitate out before the rest.
The Role of Precipitation in Wastewater Handling
Solubility equilibria are useful tools in the treatment of wastewater carried out in facilities that may treat the municipal h2o in your city or town (Figure v). Specifically, selective precipitation is used to remove contaminants from wastewater earlier it is released back into natural bodies of water. For example, phosphate ions [latex](\text{PO}_4^{\;\;2-})[/latex] are frequently nowadays in the water discharged from manufacturing facilities. An affluence of phosphate causes excess algae to grow, which impacts the amount of oxygen bachelor for marine life equally well as making water unsuitable for human consumption.

I common way to remove phosphates from water is by the addition of calcium hydroxide, known as lime, Ca(OH)2. The lime is converted into calcium carbonate, a strong base, in the h2o. As the water is made more bones, the calcium ions react with phosphate ions to produce hydroxylapatite, Cafive(PO4)3(OH), which then precipitates out of the solution:
[latex]5\text{Ca}^{2+}\;+\;iii\text{PO}_4^{\;\;3-}\;+\;\text{OH}^{-}\;{\leftrightharpoons}\;\text{Ca}_{10}(\text{PO}_4)_6{\cdot}(\text{OH})_2(s)[/latex]
The precipitate is and so removed by filtration and the h2o is brought back to a neutral pH past the improver of COtwo in a recarbonation process. Other chemicals tin also exist used for the removal of phosphates by precipitation, including iron(Iii) chloride and aluminum sulfate.
View this site for more than information on how phosphorus is removed from wastewater.
Selective atmospheric precipitation tin also be used in qualitative assay. In this method, reagents are added to an unknown chemic mixture in social club to induce precipitation. Certain reagents cause specific ions to precipitate out; therefore, the improver of the reagent tin can be used to make up one's mind whether the ion is nowadays in the solution.

View this simulation to study the process of salts dissolving and forming saturated solutions and precipitates for specific compounds, or compounds for which you select the charges on the ions and the Yard sp
Example 11
Precipitation of Silvery Halides
A solution contains 0.0010 mol of KI and 0.10 mol of KCl per liter. AgNO3 is gradually added to this solution. Which forms first, solid AgI or solid AgCl?
Solution
The two equilibria involved are:
[latex]\text{AgCl}(due south)\;{\rightleftharpoons}\;\text{Ag}^{+}(aq)\;+\;\text{Cl}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = 1.6\;\times\;10^{-10}[/latex]
[latex]\text{AgI}(s)\;{\rightleftharpoons}\;\text{Ag}^{+}(aq)\;+\;\text{I}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = 1.5\;\times\;x^{-16}[/latex]
If the solution independent virtually equal concentrations of Cl– and I–, and then the silver table salt with the smallest Grand sp (AgI) would precipitate first. The concentrations are not equal, withal, so we should find the [Ag+] at which AgCl begins to precipitate and the [Ag+] at which AgI begins to precipitate. The salt that forms at the lower [Ag+] precipitates beginning.
For AgI: AgI precipitates when Q equals K sp for AgI (1.5 × 10–16). When [I–] = 0.0010 M:
[latex]Q = [\text{Ag}^{+}][\text{I}^{-}] = [\text{Ag}^{+}](0.0010) = 1.5\;\times\;10^{-16}[/latex]
[latex][\text{Ag}^{+}] = \frac{ane.eight\;\times\;ten^{-10}}{0.10} = 1.6\;\times\;10^{-9}[/latex]
AgI begins to precipitate when [Ag+] is 1.v × 10–thirteen M.
For AgCl: AgCl precipitates when Q equals Grand sp for AgCl (i.half-dozen × 10–10). When [Cl–] = 0.10 M:
[latex]Q_{\text{sp}} = [\text{Ag}^{+}][\text{Cl}^{-}] = [\text{Ag}^{+}](0.10) = one.6\;\times\;x^{-10}[/latex]
[latex][\text{Ag}^{+}] = \frac{1.viii\;\times\;10^{-10}}{0.10} = ane.half-dozen\;\times\;10^{-9}\;M[/latex]
AgCl begins to precipitate when [Ag+] is one.6 × 10–9 M.
AgI begins to precipitate at a lower [Ag+] than AgCl, and so AgI begins to precipitate first.
Cheque Your Learning
If silverish nitrate solution is added to a solution which is 0.050 Grand in both Cl– and Br– ions, at what [Ag+] would precipitation begin, and what would be the formula of the precipitate?
Answer:
[Ag+] = 1.0 × x–11 Chiliad; AgBr precipitates kickoff
Mutual Ion Result
As we saw when nosotros discussed buffer solutions, the hydronium ion concentration of an aqueous solution of acetic acrid decreases when the strong electrolyte sodium acetate, NaCH3COtwo, is added. We can explain this effect using Le Châtelier'south principle. The addition of acetate ions causes the equilibrium to shift to the left, decreasing the concentration of [latex]\text{H}_3\text{O}^{+}[/latex] to compensate for the increased acetate ion concentration. This increases the concentration of CHthreeCO2H:
[latex]\text{CH}_3\text{CO}_2\text{H}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{CH}_3\text{CO}_2^{\;\;-}[/latex]
Because sodium acetate and acetic acid have the acetate ion in common, the influence on the equilibrium is called the common ion issue.
The mutual ion effect can also take a direct result on solubility equilibria. Suppose we are looking at the reaction where silvery iodide is dissolved:
[latex]\text{AgI}(south)\;{\rightleftharpoons}\;\text{Ag}^{+}(aq)\;+\;\text{I}^{-}(aq)[/latex]
If we were to add together potassium iodide (KI) to this solution, we would be calculation a substance that shares a mutual ion with silverish iodide. Le Châtelier's principle tells us that when a change is made to a arrangement at equilibrium, the reaction will shift to counteract that modify. In this example, there would be an excess of iodide ions, so the reaction would shift toward the left, causing more silverish iodide to precipitate out of solution.

View this simulation to see how the common ion effect work with different concentrations of salts.
Example 12
Common Ion Issue
Calculate the molar solubility of cadmium sulfide (CdS) in a 0.010-Yard solution of cadmium bromide (CdBr2). The K sp of CdS is 1.0 × 10–28.
Solution
The start thing you should notice is that the cadmium sulfide is dissolved in a solution that contains cadmium ions. Nosotros need to use an Ice tabular array to prepare upwards this problem and include the CdBr2 concentration as a contributor of cadmium ions:
[latex]\text{CdS}(due south)\;{\leftrightharpoons}\;\text{Cd}^{2+}(aq)\;+\;\text{Southward}^{2-}(aq)[/latex]
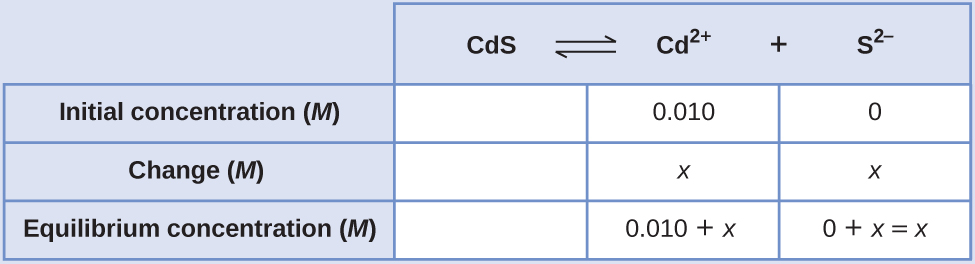
[latex]K_{\text{sp}} = [\text{Cd}^{two+}][\text{S}^{two-}] = ane.0\;\times\;10^{-28}[/latex]
[latex](0.010\;+\;x)(x) = 1.0\;\times\;x^{-28}[/latex]
[latex]x^2\;+\;0.010x\;-\;ane.0\;\times\;10^{-28} = 0[/latex]
Nosotros can solve this equation using the quadratic formula, but we can also make an assumption to make this adding much simpler. Since the Chiliad sp value is and so small compared with the cadmium concentration, we tin can assume that the change between the initial concentration and the equilibrium concentration is negligible, so that 0.010 + x ~ 0.010. Going dorsum to our K sp expression, we would now get:
[latex]K_{\text{sp}} = [\text{Cd}^{2+}][\text{S}^{2-}] = 1.0\;\times\;10^{-28}[/latex]
[latex](0.010)(x) = 1.0\;\times\;10^{-28}[/latex]
[latex]10 = 1.0\;\times\;ten^{-26}[/latex]
Therefore, the molar solubility of CdS in this solution is ane.0 × 10–26 Thou.
Bank check Your Learning
Calculate the tooth solubility of aluminum hydroxide, Al(OH)three, in a 0.015-One thousand solution of aluminum nitrate, Al(NOthree)3. The K sp of Al(OH)iii is 2 × ten–32.
Key Concepts and Summary
The equilibrium constant for an equilibrium involving the precipitation or dissolution of a slightly soluble ionic solid is called the solubility product, Chiliad sp, of the solid. When nosotros accept a heterogeneous equilibrium involving the slightly soluble solid M p 10 q and its ions Mchiliad+ and Xn–:
[latex]\text{1000}_p\text{X}_q(southward)\;{\leftrightharpoons}\;p\text{M}^{\text{m}+}(aq)\;+\;q\text{Ten}^{\text{n}-}(aq)[/latex]
Nosotros write the solubility product expression equally:
[latex]K_{\text{sp}} = [\text{M}^{\text{m}+}]^p[\text{10}^{\text{n}-}]^q[/latex]
The solubility production of a slightly soluble electrolyte can be calculated from its solubility; conversely, its solubility can exist calculated from its Yard sp, provided the only significant reaction that occurs when the solid dissolves is the formation of its ions.
A slightly soluble electrolyte begins to precipitate when the magnitude of the reaction quotient for the dissolution reaction exceeds the magnitude of the solubility product. Precipitation continues until the reaction quotient equals the solubility product.
A reagent can be added to a solution of ions to allow one ion to selectively precipitate out of solution. The mutual ion effect can also play a role in atmospheric precipitation reactions. In the presence of an ion in mutual with one of the ions in the solution, Le Châtelier'due south principle applies and more precipitate comes out of solution and then that the molar solubility is reduced.
Key Equations
- [latex]\text{M}_p\text{X}_q(s)\;{\leftrightharpoons}\;p\text{Thousand}^{\text{m}+}(aq)\;+\;q\text{X}^{\text{northward}-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{M}^{\text{m}+}]^p[\text{10}^{\text{n}-}]^q[/latex]
Chemistry Finish of Chapter Exercises
- Complete the changes in concentrations for each of the following reactions:
(a) [latex]\begin{array}{lccc} \text{AgI}(s)\;{\longrightarrow}\; & \text{Ag}^{+}(aq) & + & \text{I}^{-}(aq) \\[0.5em] & 10 & & \rule[0ex]{2.5em}{0.1ex} \terminate{array}[/latex]
(b) [latex]\begin{array}{lccc} \text{CaCO}_3(s)\;{\longrightarrow} & \text{Ca}^{2+}(aq) & + & \text{CO}_3^{\;\;two-}(aq) \\[0.5em] & \dominion[0ex]{two.5em}{0.1ex} & & ten \terminate{array}[/latex]
(c) [latex]\begin{array}{lccc} \text{Mg(OH)}_2(s)\;{\longrightarrow} & \text{Mg}^{2+}(aq) & + & 2\text{OH}^{-}(aq) \\[0.5em] & x & & \rule[0ex]{2.5em}{0.1ex} \end{array}[/latex]
(d) [latex]\begin{assortment}{lccc} \text{Mg}_3(\text{PO}_4)_2(due south)\;{\longrightarrow} & three\text{Mg}^{2+}(aq) & + & ii\text{PO}_4^{\;\;3-}(aq) \\[0.5em] & \rule[0ex]{2.5em}{0.1ex} & & 2x \cease{array}[/latex]
(e) [latex]\brainstorm{array}{lccccc} \text{Ca}_5(\text{PO}_4)_3\text{OH}(s)\;{\longrightarrow} & five\text{Ca}^{ii+}(aq) & + & iii\text{PO}_4^{\;\;3-}(aq) & + & \text{OH}^{-}(aq) \\[0.5em] & \rule[0ex]{2.5em}{0.1ex} & & \rule[0ex]{ii.5em}{0.1ex} & & x \end{array}[/latex]
- Complete the changes in concentrations for each of the following reactions:
(a) [latex]\begin{array}{lccc} \text{BaSO}_4(s)\;{\longrightarrow} & \text{Ba}^{2+}(aq) & + & \text{SO}_4^{\;\;two-}(aq) \\[0.5em] & ten & & \dominion[0ex]{two.5em}{0.1ex} \stop{array}[/latex]
(b) [latex]\begin{array}{lccc} \text{Ag}_2\text{SO}_4(s)\;{\longrightarrow} & 2\text{Ag}^{+}(aq) & + & \text{SO}_4^{\;\;2-}(aq) \\[0.5em] & \rule[0ex]{2.5em}{0.1ex} & & x \terminate{array}[/latex]
(c) [latex]\begin{array}{lccc} \text{Al(OH)}_3(due south)\;{\longrightarrow} & \text{Al}^{iii+}(aq) & + & 3\text{OH}^{-}(aq) \\[0.5em] & ten & & \rule[0ex]{2.5em}{0.1ex} \end{assortment}[/latex]
(d) [latex]\begin{assortment}{lccccc} \text{Lead(OH)Cl}(s)\;{\longrightarrow} & \text{Pb}^{ii+}(aq) & + & \text{OH}^{-}(aq) & + & \text{Cl}^{-}(aq) \\[0.5em] & \rule[0ex]{2.5em}{0.1ex} & & x & & \rule[0ex]{ii.5em}{0.1ex} \end{assortment}[/latex]
(east) [latex]\begin{assortment}{lccc} \text{Ca}_3(\text{AsO}_4)_2(due south)\;{\longrightarrow} & 3\text{Ca}^{2+}(aq) & + & two\text{AsO}_4^{\;\;3-}(aq) \\[0.5em] & 3x & & \dominion[0ex]{two.5em}{0.1ex} \stop{array}[/latex]
- How practice the concentrations of Ag+ and [latex]\text{CrO}_4^{\;\;2-}[/latex] in a saturated solution above ane.0 g of solid AgtwoCrOfour change when 100 g of solid Ag2CrO4 is added to the arrangement? Explain.
- How do the concentrations of Leadtwo+ and Southward2– alter when One thousand2Southward is added to a saturated solution of PbS?
- What additional data exercise nosotros need to answer the following question: How is the equilibrium of solid silver bromide with a saturated solution of its ions affected when the temperature is raised?
- Which of the post-obit slightly soluble compounds has a solubility greater than that calculated from its solubility product because of hydrolysis of the anion present: CoSO3, CuI, PbCO3, PbClii, TliiDue south, KClO4?
- Which of the following slightly soluble compounds has a solubility greater than that calculated from its solubility product because of hydrolysis of the anion present: AgCl, BaSO4, CaF2, Hg2I2, MnCO3, ZnS, PbS?
- Write the ionic equation for dissolution and the solubility product (Thou sp) expression for each of the post-obit slightly soluble ionic compounds:
(a) PbCl2
(b) Ag2Due south
(c) Sriii(PO4)ii
(d) SrSOfour
- Write the ionic equation for the dissolution and the Thousand sp expression for each of the following slightly soluble ionic compounds:
(a) LaFthree
(b) CaCOthree
(c) Ag2SO4
(d) Atomic number 82(OH)2
- The Handbook of Chemistry and Physics gives solubilities of the following compounds in grams per 100 mL of water. Because these compounds are only slightly soluble, assume that the volume does not change on dissolution and calculate the solubility product for each.
(a) BaSiF6, 0.026 g/100 mL (contains [latex]\text{SiF}_6^{\;\;2-}[/latex] ions)
(b) Ce(IO3)4, one.5 × 10–two grand/100 mL
(c) Gd2(So4)3, three.98 g/100 mL
(d) (NH4)iiPtBrhalf-dozen, 0.59 thousand/100 mL (contains [latex]\text{PtBr}_6^{\;\;2-}[/latex] ions)
- The Handbook of Chemistry and Physics gives solubilities of the following compounds in grams per 100 mL of h2o. Because these compounds are but slightly soluble, assume that the volume does not alter on dissolution and calculate the solubility product for each.
(a) BaSeO4, 0.0118 m/100 mL
(b) Ba(BrOthree)two·H2O, 0.30 m/100 mL
(c) NH4MgAsOiv·6H2O, 0.038 g/100 mL
(d) La2(MoOiv)3, 0.00179 g/100 mL
- Use solubility products and predict which of the following salts is the most soluble, in terms of moles per liter, in pure water: CaFii, HgiiCl2, PbI2, or Sn(OH)two.
- Assuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product:
(a) KHC4H4Ohalf-dozen
(b) PbIii
(c) Ag4[Fe(CN)6], a common salt containing the [latex]\text{Fe(CN)}_4^{\;\;-}[/latex] ion
(d) Hg2I2
- Assuming that no equilibria other than dissolution are involved, summate the molar solubility of each of the following from its solubility product:
(a) Ag2SO4
(b) PbBr2
(c) AgI
(d) CaC2Oiv·H2O
- Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solutions of salts in contact with a solution containing a common ion. Show that changes in the initial concentrations of the common ions can be neglected.
(a) AgCl(south) in 0.025 M NaCl
(b) CaFii(south) in 0.00133 M KF
(c) Ag2SO4(s) in 0.500 L of a solution containing 19.50 k of GrandtwoSo4
(d) Zn(OH)2(s) in a solution buffered at a pH of 11.45
- Bold that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the post-obit solutions of salts in contact with a solution containing a common ion. Bear witness that changes in the initial concentrations of the common ions can be neglected.
(a) TlCl(s) in 1.250 M HCl
(b) PbI2(s) in 0.0355 M CaI2
(c) Ag2CrOfour(s) in 0.225 L of a solution containing 0.856 g of 10002CrOiv
(d) Cd(OH)ii(s) in a solution buffered at a pH of 10.995
- Bold that no equilibria other than dissolution are involved, summate the concentration of all solute species in each of the post-obit solutions of salts in contact with a solution containing a common ion. Show that it is non appropriate to neglect the changes in the initial concentrations of the mutual ions.
(a) TlCl(s) in 0.025 M TlNOthree
(b) BaFii(s) in 0.0313 M KF
(c) MgCiiO4 in two.250 L of a solution containing viii.156 g of Mg(NO3)2
(d) Ca(OH)2(south) in an unbuffered solution initially with a pH of 12.700
- Explain why the changes in concentrations of the common ions in Affiliate 15.1 Chemistry End of Affiliate Exercise 17 can be neglected.
- Explain why the changes in concentrations of the mutual ions in Affiliate 15.1 Chemistry Stop of Affiliate Exercise 17 cannot exist neglected.
- Calculate the solubility of aluminum hydroxide, Al(OH)3, in a solution buffered at pH 11.00.
- Refer to Appendix J for solubility products for calcium salts. Determine which of the calcium salts listed is near soluble in moles per liter and which is most soluble in grams per liter.
- Most barium compounds are very poisonous; however, barium sulfate is oftentimes administered internally as an aid in the X-ray examination of the lower intestinal tract (Figure iii). This apply of BaSOiv is possible considering of its low solubility. Summate the molar solubility of BaSO4 and the mass of barium present in ane.00 L of water saturated with BaSOiv.
- Public Health Service standards for drinking water ready a maximum of 250 mg/L (2.60 × ten–3 M) of [latex]\text{Then}_4^{\;\;2-}[/latex] because of its cathartic action (information technology is a laxative). Does natural water that is saturated with CaSOiv ("gyp" water) as a result or passing through soil containing gypsum, CaSO4·2HtwoO, see these standards? What is [latex]\text{SO}_4^{\;\;two-}[/latex] in such water?
- Perform the following calculations:
(a) Summate [Ag+] in a saturated aqueous solution of AgBr.
(b) What will [Ag+] exist when plenty KBr has been added to make [Br–] = 0.050 M?
(c) What will [Br–] be when plenty AgNO3 has been added to make [Ag+] = 0.020 M?
- The solubility production of CaSOfour·2HtwoO is two.iv × 10–5. What mass of this salt will deliquesce in 1.0 Fifty of 0.010 G [latex]\text{Then}_4^{\;\;ii-}[/latex]?
- Assuming that no equilibria other than dissolution are involved, summate the concentrations of ions in a saturated solution of each of the following (run across Appendix J for solubility products).
(a) TlCl
(b) BaF2
(c) AgiiCrO4
(d) CaC2Ofour·H2O
(e) the mineral anglesite, PbSO4
- Assuming that no equilibria other than dissolution are involved, calculate the concentrations of ions in a saturated solution of each of the post-obit (see Appendix J for solubility products):
(a) AgI
(b) AgtwoSo4
(c) Mn(OH)2
(d) Sr(OH)two·8HiiO
(e) the mineral brucite, Mg(OH)2
- The following concentrations are found in mixtures of ions in equilibrium with slightly soluble solids. From the concentrations given, calculate K sp for each of the slightly soluble solids indicated:
(a) AgBr: [Ag+] = 5.7 × 10–vii Thou, [Br–] = 5.vii × 10–vii M
(b) CaCO3: [Catwo+] = five.3 × ten–3 M, [latex][\text{CO}_3^{\;\;2-}][/latex] = 9.0 × 10–7 Thou
(c) PbF2: [Lead2+] = ii.i × 10–3 Thousand, [F–] = four.2 × 10–3 M
(d) Ag2CrOiv: [Ag+] = 5.3 × 10–v Thou, 3.2 × 10–3 M
(eastward) InF3: [Inthree+] = 2.3 × 10–3 G, [F–] = vii.0 × ten–iii Chiliad
- The post-obit concentrations are found in mixtures of ions in equilibrium with slightly soluble solids. From the concentrations given, calculate K sp for each of the slightly soluble solids indicated:
(a) TlCl: [Tl+] = 1.21 × 10–2 Thousand, [Cl–] = one.two × 10–2 M
(b) Ce(IOiii)four: [Ce4+] = 1.8 × 10–iv Yard, [latex][\text{IO}_3^{\;\;-}][/latex] = 2.6 × 10–xiii M
(c) Gdtwo(And then4)3: [Gd3+] = 0.132 M, [latex][\text{And so}_4^{\;\;ii-}][/latex] = 0.198 Yard
(d) Ag2SOiv: [Ag+] = 2.40 × 10–2 M, [latex][\text{And then}_4^{\;\;ii-}][/latex] = 2.05 × 10–2 One thousand
(e) BaSOfour: [Ba2+] = 0.500 One thousand, [latex][\text{SO}_4^{\;\;two-}][/latex] = two.xvi × 10–10 Thou
- Which of the post-obit compounds precipitates from a solution that has the concentrations indicated? (Run across Appendix J for Grand sp values.)
(a) KClO4: [K+] = 0.01 M, [latex][\text{ClO}_4^{\;\;-}][/latex] = 0.01 K
(b) KiiPtClsix: [One thousand+] = 0.01 M, [latex][\text{PtCl}_6^{\;\;2-}][/latex] = 0.01 Thousand
(c) PbItwo: [Atomic number 82ii+] = 0.003 Grand, [I–] = i.3 × x–3 G
(d) AgiiS: [Ag+] = 1 × 10–ten One thousand, [Due south2–] = 1 × 10–thirteen M
- Which of the following compounds precipitates from a solution that has the concentrations indicated? (See Appendix J for K sp values.)
(a) CaCOthree: [Ca2+] = 0.003 M, [latex][\text{CO}_3^{\;\;two-}][/latex] = 0.003 Thou
(b) Co(OH)2: [Cotwo+] = 0.01 M, [OH–] = i × 10–seven M
(c) CaHPO4: [Ca2+] = 0.01 Thou, [latex][\text{HPO}_4^{\;\;2-}][/latex] = 2 × 10–half-dozen Grand
(d) Pbiii(POfour)two: [Leadii+] = 0.01 M, [latex][\text{PO}_4^{\;\;3-}][/latex] = 1 × 10–13 M
- Calculate the concentration of Tl+ when TlCl but begins to precipitate from a solution that is 0.0250 M in Cl–.
- Calculate the concentration of sulfate ion when BaSOfour but begins to precipitate from a solution that is 0.0758 M in Ba2+.
- Summate the concentration of Sr2+ when SrFii starts to precipitate from a solution that is 0.0025 M in F–.
- Calculate the concentration of [latex]\text{PO}_4^{\;\;iii-}[/latex] when Ag3PO4 starts to precipitate from a solution that is 0.0125 M in Ag+.
- Calculate the concentration of F– required to begin atmospheric precipitation of CaF2 in a solution that is 0.010 G in Ca2+.
- Calculate the concentration of Ag+ required to begin precipitation of Ag2CO3 in a solution that is 2.50 × ten–6 M in [latex]\text{CO}_3^{\;\;2-}[/latex].
- What [Ag+] is required to reduce [latex][\text{CO}_3^{\;\;2-}][/latex] to eight.2 × x–4 M by atmospheric precipitation of Ag2CO3?
- What [F–] is required to reduce [Ca2+] to 1.0 × 10–4 M past precipitation of CaF2?
- A book of 0.800 Fifty of a 2 × 10–four–G Ba(NO3)2 solution is added to 0.200 L of 5 × 10–4 M Li2Then4. Does BaSO4 precipitate? Explain your respond.
- Perform these calculations for nickel(Ii) carbonate. (a) With what volume of h2o must a precipitate containing NiCO3 be washed to dissolve 0.100 yard of this chemical compound? Assume that the launder water becomes saturated with NiCO3 (K sp = one.36 × x–vii).
(b) If the NiCO3 were a contaminant in a sample of CoCOiii (Yard sp = i.0 × 10–12), what mass of CoCOiii would accept been lost? Go along in mind that both NiCO3 and CoCO3 deliquesce in the same solution.
- Atomic number 26 concentrations greater than 5.4 × 10–6 G in water used for laundry purposes tin cause staining. What [OH–] is required to reduce [Atomic number 26ii+] to this level by atmospheric precipitation of Fe(OH)2?
- A solution is 0.010 M in both Cutwo+ and Cd2+. What percentage of Cdtwo+ remains in the solution when 99.9% of the Cu2+ has been precipitated as CuS by calculation sulfide?
- A solution is 0.fifteen One thousand in both Lead2+ and Ag+. If Cl– is added to this solution, what is [Ag+] when PbCl2 begins to precipitate?
- What reagent might be used to separate the ions in each of the following mixtures, which are 0.1 M with respect to each ion? In some cases it may be necessary to command the pH. (Hint: Consider the G sp values given in Appendix J.)
(a) [latex]\text{Hg}_2^{\;\;2+}[/latex] and Cu2+
(b) [latex]\text{And then}_4^{\;\;ii-}[/latex] and Cl–
(c) Hg2+ and Co2+
(d) Zntwo+ and Srtwo+
(east) Batwo+ and Mg2+
(f) [latex]\text{CO}_3^{\;\;2-}[/latex] and OH–
- A solution contains ane.0 × 10–five mol of KBr and 0.10 mol of KCl per liter. AgNO3 is gradually added to this solution. Which forms first, solid AgBr or solid AgCl?
- A solution contains 1.0 × 10–2 mol of KI and 0.10 mol of KCl per liter. AgNO3 is gradually added to this solution. Which forms first, solid AgI or solid AgCl?
- The calcium ions in human claret serum are necessary for coagulation (Figure four). Potassium oxalate, K2C2Ofour, is used as an anticoagulant when a blood sample is drawn for laboratory tests considering it removes the calcium as a precipitate of CaCtwoOfour·H2O. It is necessary to remove all but 1.0% of the Ca2+ in serum in order to forestall coagulation. If normal blood serum with a buffered pH of seven.xl contains nine.5 mg of Ca2+ per 100 mL of serum, what mass of Chiliad2C2O4 is required to prevent the coagulation of a 10 mL claret sample that is 55% serum by volume? (All volumes are accurate to two meaning figures. Note that the book of serum in a 10-mL blood sample is v.5 mL. Assume that the Ksp value for CaC2O4 in serum is the same as in water.)
- Near 50% of urinary calculi (kidney stones) consist of calcium phosphate, Caiii(PO4)2. The normal mid range calcium content excreted in the urine is 0.10 g of Ca2+ per twenty-four hour period. The normal mid range amount of urine passed may be taken equally 1.four Fifty per day. What is the maximum concentration of phosphate ion that urine can contain before a calculus begins to course?
- The pH of normal urine is half dozen.xxx, and the total phosphate concentration [latex]([\text{PO}_4^{\;\;three-}]\;+\;[\text{HPO}_4^{\;\;two-}]\;+\;[\text{H}_2\text{PO}_4^{\;\;-}]\;+\;[\text{H}_3\text{PO}_4])[/latex] is 0.020 M. What is the minimum concentration of Ca2+ necessary to induce kidney stone formation? (See Chemistry End of Chapter Exercise 49 for additional data.)
- Magnesium metal (a component of alloys used in aircraft and a reducing agent used in the production of uranium, titanium, and other active metals) is isolated from sea water by the following sequence of reactions:
[latex]\text{Mg}^{2+}(aq)\;+\;\text{Ca(OH)}_2(aq)\;{\longrightarrow}\;\text{Mg(OH)}_2(south)\;+\;\text{Ca}^{2+}(aq)[/latex]
[latex]\text{Mg(OH)}_2(due south)\;+\;2\text{HCl}(aq)\;{\longrightarrow}\;\text{MgCl}_2(s)\;+\;two\text{H}_2\text{O}(l)[/latex]
[latex]\text{MgCl}_2(l)\;{\xrightarrow{\text{electrolysis}}}\;\text{Mg}(south)\;+\;\text{Cl}_2(1000)[/latex]
Body of water water has a density of 1.026 g/cmiii and contains 1272 parts per million of magnesium as Mgii+(aq) past mass. What mass, in kilograms, of Ca(OH)2 is required to precipitate 99.nine% of the magnesium in 1.00 × ten3 L of sea water?
- Hydrogen sulfide is bubbled into a solution that is 0.10 M in both Pbtwo+ and Ironii+ and 0.xxx M in HCl. After the solution has come to equilibrium it is saturated with H2S ([H2S] = 0.10 M). What concentrations of Atomic number 822+ and Fe2+ remain in the solution? For a saturated solution of HiiSouthward we can use the equilibrium:
[latex]\text{H}_2\text{S}(aq)\;+\;ii\text{H}_2\text{O}(l)\;{\leftrightharpoons}\;2\text{H}_3\text{O}^{+}(aq)\;+\;\text{S}^{2-}(aq)\;\;\;\;\;\;\;Thou = 1.0\;\times\;ten^{-26}[/latex]
(Hint: The [latex][\text{H}_3\text{O}^{+}][/latex] changes every bit metal sulfides precipitate.)
- Perform the following calculations involving concentrations of iodate ions:
(a) The iodate ion concentration of a saturated solution of La(IO3)three was found to exist 3.1 × ten–3 mol/L. Detect the M sp.
(b) Find the concentration of iodate ions in a saturated solution of Cu(IOiii)ii (K sp = vii.4 × 10–eight).
- Summate the tooth solubility of AgBr in 0.035 M NaBr (Grand sp = five × ten–13).
- How many grams of Atomic number 82(OH)2 will dissolve in 500 mL of a 0.050-M PbCl2 solution (Grand sp = 1.2 × 10–15)?
- Use the simulation from the earlier Link to Learning to consummate the following practise:. Using 0.01 m CaF2, give the Grandsp values found in a 0.2-M solution of each of the salts. Discuss why the values change as you change soluble salts.
- How many grams of Milk of Magnesia, Mg(OH)2 (south) (58.three m/mol), would exist soluble in 200 mL of water. One thousand sp = 7.i × ten–12. Include the ionic reaction and the expression for Thousand sp in your reply. (K w = 1 × x–14 = [H3O+][OH–])
- 2 hypothetical salts, LM2 and LQ, have the same molar solubility in HtwoO. If K sp for LMtwo is 3.twenty × 10–v, what is the Thou sp value for LQ?
- Which of the post-obit carbonates will class get-go? Which of the following will form last? Explain.
(a) [latex]\text{MgCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = 3.5\;\times\;x^{-8}[/latex]
(b) [latex]\text{CaCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = iv.ii\;\times\;10^{-7}[/latex]
(c) [latex]\text{SrCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = 3.9\;\times\;10^{-9}[/latex]
(d) [latex]\text{BaCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = 4.4\;\times\;10^{-v}[/latex]
(east) [latex]\text{MnCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = 5.ane\;\times\;ten^{-nine}[/latex]
- How many grams of Zn(CN)2(s) (117.44 one thousand/mol) would be soluble in 100 mL of H2O? Include the balanced reaction and the expression for K sp in your answer. The K sp value for Zn(CN)2(s) is iii.0 × 10–16.
Glossary
- common ion effect
- result on equilibrium when a substance with an ion in common with the dissolved species is added to the solution; causes a subtract in the solubility of an ionic species, or a decrease in the ionization of a weak acid or base
- molar solubility
- solubility of a compound expressed in units of moles per liter (mol/50)
- selective precipitation
- procedure in which ions are separated using differences in their solubility with a given precipitating reagent
- solubility product (1000 sp)
- equilibrium constant for the dissolution of a slightly soluble electrolyte
Solutions
Answers to Chemistry End of Chapter Exercises
1.
(a) [latex]\begin{array}{lccc} \text{AgI}(southward)\;{\rightleftharpoons}\; & \text{Ag}^{+}(aq) & + & \text{I}^{-}(aq) \\[0.5em] & x & & \rule[-0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}x \end{array}[/latex]
(b) [latex]\brainstorm{assortment}{lccc} \text{CaCO}_3(s)\;{\rightleftharpoons} & \text{Ca}^{2+}(aq) & + & \text{CO}_3^{\;\;2-}(aq) \\[0.5em] & \rule[-0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}x & & x \stop{array}[/latex]
(c) [latex]\begin{array}{lccc} \text{Mg(OH)}_2(s)\;{\rightleftharpoons} & \text{Mg}^{2+}(aq) & + & ii\text{OH}^{-}(aq) \\[0.5em] & x & & \dominion[-0.25ex]{1em}{0.1ex}\hspace{-1em}2x \cease{assortment}[/latex]
(d) [latex]\begin{assortment}{lccc} \text{Mg}_3(\text{PO}_4)_2(s)\;{\rightleftharpoons} & 3\text{Mg}^{two+}(aq) & + & 2\text{PO}_4^{\;\;iii-}(aq) \\[0.5em] & \rule[-0.25ex]{1em}{0.1ex}\hspace{-1em}3x & & 2x \end{array}[/latex]
(due east) [latex]\begin{array}{lccccc} \text{Ca}_5(\text{PO}_4)_3\text{OH}(southward)\;{\rightleftharpoons} & 5\text{Ca}^{2+}(aq) & + & 3\text{PO}_4^{\;\;three-}(aq) & + & \text{OH}^{-}(aq) \\[0.5em] & \rule[-0.25ex]{1em}{0.1ex}\hspace{-1em}5x & & \rule[-0.25ex]{1em}{0.1ex}\hspace{-1em}3x & & x \end{assortment}[/latex]
3. At that place is no modify. A solid has an activeness of 1 whether there is a little or a lot.
5. The solubility of silver bromide at the new temperature must be known. Unremarkably the solubility increases and some of the solid silver bromide volition dissolve.
7. CaF2, MnCOthree, and ZnS
nine. (a) [latex]\text{LaF}_3(s)\;{\rightleftharpoons}\;\text{La}^{3+}(aq)\;+\;three\text{F}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{La}^{iii+}][\text{F}^{-}]^three[/latex];
(b) [latex]\text{CaCO}_3(southward)\;{\rightleftharpoons}\;\text{Ca}^{two+}(aq)\;+\;\text{CO}_3^{\;\;2-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ca}^{ii+}][\text{CO}_3^{\;\;2-}][/latex];
(c) [latex]\text{Ag}_2\text{SO}_4(s)\;{\rightleftharpoons}\;two\text{Ag}^{+}(aq)\;+\;\text{SO}_4^{\;\;2-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ag}^{+}]^2[\text{Then}_4^{\;\;2-}][/latex];
(d) [latex]\text{Atomic number 82(OH)}_2(south)\;{\rightleftharpoons}\;\text{Atomic number 82}^{2+}(aq)\;+\;2\text{OH}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Lead}^{2+}][\text{OH}^{-}]^2[/latex]
11. (a)1.77 × 10–seven; (b) 1.vi × 10–half-dozen; (c) two.2 × 10–9; (d) 7.91 × 10–22
13. (a) 2 × 10–2 M; (b) 1.5 × x–3 1000; (c) 2.27 × 10–9 Thousand; (d) two.2 × 10–10 K
15. (a) 6.4 × 10−9 M = [Ag+], [Cl−] = 0.025 Grand
Check: [latex]\frac{6.4\;\times\;10^{-9}\;Chiliad}{0.025\;Yard}\;\times\;100\% = 2.half dozen\;\times\;10^{-5}\;%[/latex],an insignificant change;
(b) two.2 × 10−5 M = [Ca2+], [F−] = 0.0013 Thou
Bank check: [latex]\frac{2.26\;\times\;10^{-5}\;M}{0.00133\;K}\;\times\;100\% = one.70\%[/latex]. This value is less than 5% and can be ignored.
(c) 0.2238 M = [latex][\text{SO}_4^{\;\;two-}][/latex]; [Ag+] = seven.4 × ten–iii K
Check: [latex]\frac{3.7\;\times\;ten^{-three}}{0.2238}\;\times\;100\% = ane.64\;\times\;10^{-2}\%[/latex]; the condition is satisfied.
(d) [OH–] = 2.8 × ten–three M; 5.seven × 10−12 G = [Zn2+]
Check: [latex]\frac{v.7\;\times\;x^{-12}}{2.eight\;\times\;x^{-three}}\;\times\;100\% = 2.0\;\times\;10^{-vii}\%[/latex]; 10 is less than 5% of [OH–] and is, therefore, negligible.
17. (a) [Cl–] = 7.six × 10−3 Grand
Cheque: [latex]\frac{7.six\;\times\;10^{-three}}{0.025}\;\times\;100\% = 30\%[/latex]
This value is as well big to drop x. Therefore solve by using the quadratic equation:
[Ti+] = 3.one × 10–2 M
[Cl–] = 6.one × 10–iii
(b) [Baii+] = seven.7 × 10–4 1000
Check: [latex]\frac{7.7\;\times\;x^{-4}}{0.0313}\;\times\;100\% = 2.4\%[/latex]
Therefore, the condition is satisfied.
[Batwo+] = 7.7 × 10–4 M
[F–] = 0.0321 M;
(c) Mg(NO3)two = 0.02444 M
[latex][\text{C}_2\text{O}_4^{\;\;ii-}] = 2.9\;\times\;x^{-5}[/latex]
Cheque: [latex]\frac{2.ix\;\times\;10^{-five}}{0.02444}\;\times\;100\% = 0.12\%[/latex]
The condition is satisfied; the above value is less than 5%.
[latex][\text{C}_2\text{O}_4^{\;\;two-}] = 2.9\;\times\;10^{-v}\;M[/latex]
[Mgii+] = 0.0244 Thou
(d) [OH–] = 0.0501 M
[Ca2+] = 3.xv × ten–3
Check: [latex]\frac{iii.xv\;\times\;10^{-three}}{0.050}\;\times\;100\% = half dozen.28\%[/latex]
This value is greater than 5%, and then a more exact method, such as successive approximations, must be used.
[Ca2+] = 2.8 × 10–three Yard
[OH–] = 0.053 × x–2 Chiliad
19. The changes in concentration are greater than 5% and thus exceed the maximum value for disregarding the alter.
21. CaSO4∙2HtwoO is the nigh soluble Ca salt in mol/L, and it is also the well-nigh soluble Ca salt in g/L.
23. 4.viii × 10–three M = [latex][\text{Then}_4^{\;\;2-}][/latex] = [Catwo+]; Since this concentration is higher than ii.60 × x–3 M, "gyp" water does non meet the standards.
25. Mass (CaSO4·2H2O) = 0.72 k/L
27. (a) [Ag+] = [I–] = 1.3 × 10–5 One thousand; (b) [Ag+] = 2.88 × 10–2 Thousand, [latex][\text{SO}_4^{\;\;2-}][/latex] = 1.44 × 10–2 Thou; (c) [Mn2+] = 3.seven × x–v Yard, [OH–] = 7.4 × ten–5 G; (d) [Srtwo+] = 4.iii × 10–two G, [OH–] = 8.6 × 10–two K; (e) [Mgii+] = 1.3 × 10–4 One thousand, [OH–] = ii.6 × 10–4 M.
29. (a) 2.0 × ten–4; (b) 5.i × 10–17; (c) one.35 × 10–4; (d) 1.18 × 10–five; (eastward) 1.08 × x–ten
31. (a) CaCO3 does precipitate.
(b) The compound does not precipitate.
(c) The compound does not precipitate.
(d) The chemical compound precipitates.
33. 3.03 × ten−seven M
35. 9.2 × 10−13 M
37. [Ag+] = ane.viii × x–3 Thou
39. 6.3 × 10–4
41. (a) 2.25 L; (b) seven.two × x–seven g
43. 100% of information technology is dissolved
45. (a) [latex]\text{Hg}_2^{\;\;2+}[/latex] and Cu2+: Add [latex]\text{SO}_4^{\;\;2-}[/latex].
(b) [latex]\text{SO}_4^{\;\;2-}[/latex] and Cl–: Add Ba2+.
(c) Hg2+ and Co2+: Add together Sii–.
(d) Znii+ and Srtwo+: Add together OH– until [OH–] = 0.050 1000.
(e) Baii+ and Mgtwo+: Add together [latex]\text{SO}_4^{\;\;2-}[/latex].
(f) [latex]\text{CO}_3^{\;\;2-}[/latex] and OH–: Add together Batwo+.
47. AgI will precipitate beginning.
49. 1.5 × 10−12 M
51. three.99 kg
53. (a) 3.1 × 10–11; (b) [Cu2+] = two.6 × 10–3; [latex][\text{IO}_3^{\;\;-}][/latex] = 5.3 × 10–3
55. ane.viii × x–5 thou Pb(OH)two
57. [latex]\text{Mg(OH)}_2(s)\;{\rightleftharpoons}\;\text{Mg}^{2+}\;+\;2\text{OH}^{-}\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Mg}^{2+}][\text{OH}^{-}]^2[/latex]
1.23 × 10−3 g Mg(OH)2
59. MnCOiii will form first, since it has the smallest K sp value it is the least soluble. MnCO3 will be the last to precipitate, it has the largest 1000 sp value.
Source: https://opentextbc.ca/chemistry/chapter/15-1-precipitation-and-dissolution/
Post a Comment for "Art B Why Does the Addition of Acid Increase the Solubility of Calcium Phosphate? Hints"